2025-09-04 – Press Releases – www.prnewswire.com
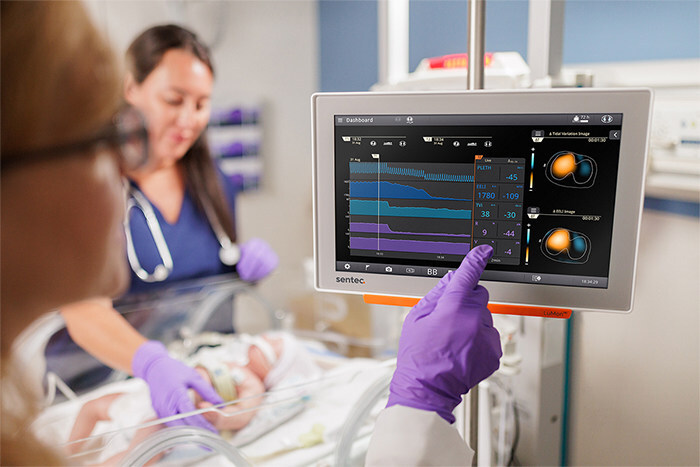
The LuMon System is also CE-marked under EU MDR and registered with TGA in Australia. LINCOLN, R.I., Sept. 4, 2025 /PRNewswire/ — Sentec, a global leader in non-invasive patient monitoring, announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for…